 Low Dose Naltrexone LDN (Part 2)
Low Dose Naltrexone LDN (Part 2)
by Jeffrey Dach MD
The Latest Medical Scandal and Outrage
In part one of this series, we discussed a novel drug treatment called LDN (low dose naltrexone), useful in treatment of Multiple Sclerosis, inflammatory bowel disease and a variety of auto-immune diseases. In part two, this article takes an in-depth look at LDN as a treatment for cancer. In addition, we review an LDN book, The Promise of Low Dose Naltrexone Therapy by Elaine Moore and Samantha Wilkinson.
Upper Left Image: molecular structure of LDN courtesy of wikimedia commons.
The Greatest Medical Discovery of the Century
No doubt, future medical history books will look back and comment that the greatest medical discovery of the 20th century was the discovery of the opiate receptor system in the brain, and endogenous opioids called endorphins. Playing a key role in this discovery is the beneficial effect of Low Dose Naltrexone, an opiate blocker which can produce a rebound-like increase in endorphin production.
This largely ignored and inexpensive off-patent drug called LDN has been used with great success over the past 20 years by various renegade physicians to cure or induce remissions in a host of seemingly unrelated diseases such as Multiple Sclerosis, Crohn's, Systemic Lupus, Rheumatoid Arthritis, Pancreatic Cancer and Lymphoma. LDN has also been found useful in Autism, and halts progression to opportunistic infection in AIDS patients.
Criticism from Mainstream Medicine
Paradoxically, LDN's ability to benefit so many seemingly unrelated medical conditions has been the greatest criticism from conventional mainstream medicine. If it sounds too good to be true, it probably is. However, since LDN is an FDA approved drug, off label use is perfectly legal, and is a common practice in mainstream medicine. LDN has virtually no adverse side effects, and based on my own short clinical experience prescribing LDN for Multiple Sclerosis, Crohn's and Ulcerative Colitis, I can report that it is amazingly effective. Even though it may sound too good to be true, in the case of LDN, I can assure you that, yes, it is all true.
Domination by Pharmaceutical Industry
 The medical system's domination by the Pharmaceutical industry is clearly apparent by the scandalous and outrageous manner in which LDN has been ignored. Mainstream neurologists refuse to prescribe LDN for multiple sclerosis, instead using prednisone, and other useless medications. Mainstream gastero-enterologists refuse to prescribe LDN for Crohn's and Ulcerative colitis, instead using prednisone, methotrexate and newer drugs like Remicade to inhibit the immune system, all with horrendous adverse side effects. Conventional oncologist refuse to prescribe LDN for cancer patients, preferring the more traditional chemotherapy, radiation and surgery, modalities which have changed very little over the past 60 years and although effective for a few selected cancers, largely ineffective for the vast majority of cancers and their relentless spread as metastatic disease.
The medical system's domination by the Pharmaceutical industry is clearly apparent by the scandalous and outrageous manner in which LDN has been ignored. Mainstream neurologists refuse to prescribe LDN for multiple sclerosis, instead using prednisone, and other useless medications. Mainstream gastero-enterologists refuse to prescribe LDN for Crohn's and Ulcerative colitis, instead using prednisone, methotrexate and newer drugs like Remicade to inhibit the immune system, all with horrendous adverse side effects. Conventional oncologist refuse to prescribe LDN for cancer patients, preferring the more traditional chemotherapy, radiation and surgery, modalities which have changed very little over the past 60 years and although effective for a few selected cancers, largely ineffective for the vast majority of cancers and their relentless spread as metastatic disease.
Upper Left Image: Naltrexone TAblet 50 mg size.

Above Left
Image: Liberty Leading the People by Delacroix (1830) Oil on canvas.
Painting in the Musée du Louvre Paris, France Commemorating the French
Revolution. Courtesy of Wikimedia Commons.
A Mass Movement Driven by People, Not Pharmaceutical Corporations
An amazing thing is happening. In spite of lack of research funding by big pharma, a mass people power movement on the internet is creating a tidal wave of interest in LDN. A flood of anecdotal reports of LDN remission and cures have been posted in the internet, and in some cases published in the medical literature. A series of LDN conferences have been organized, and have been highly successful by posting videos of the presentations on You-Tube. Publication of successful clinical trials of LDN in Crohn's disease by Jill Smith MD at Penn State University Hospital has blazed the trail for a whole series of new clinical trials driven by people power and mass support. A long list of dedicated volunteers have worked selflessly to promote new clinical trials, to get the word out about LDN and to make a reality the eventual acceptance by mainstream medicine.
October 2008 Conference at USC Medical Center
LDN for Cancer -Dr Burton Berkson
At the October 2008 LDN conference at the USC Medical Center, Burton Berkson MD PhD presented his clinical experience with LDN.
A You Tube video presentation can be viewed here.
Dr Berkson presents cases of successful remission from pancreatic cancer and B Cell lymphoma with LDN. Berkson also reported dramatic responses in cases of autoimmune diseases such as Rheumatoid Arthritis, Systemic Lupus and Dermatomyositis, as well as multiple sclerosis and inflammatory bowel disease.
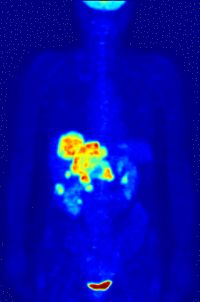
Left Image is a Pet Scan: F-18 FDG wholebody PET acquisition, showing abnormal uptake in the region of the stomach. Normal isotope levels are seen in the brain, renal collection systems, and bladder. Courtesy of Wikimedia Commons.
Lymphoma and LDN
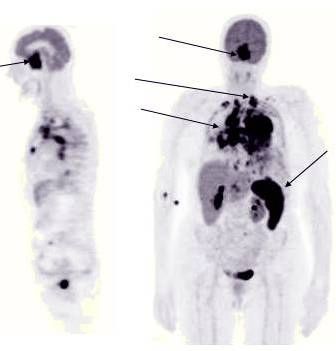
Left Image: PET Scan showing increased uptake in neoplastic deposits. (arrows)
One case report involves a lymphoma patient who experienced dramatic regression with LDN. This report by Berkson describes the treatment of a 61-year old man with biopsy-proven Lymphoma. His initial physical examination and PET/CT scan showed multiple large, metabolically active, pathologic lymph nodes that demonstrated complete resolution within 6 months of commencing therapy with Low Dose Naltrexone, taken as a capsule before sleep every night. The Pet Scans before and after LDN treatment showing regression of lymphoma can be viewed here.
Pancreatic Cancer and LDN
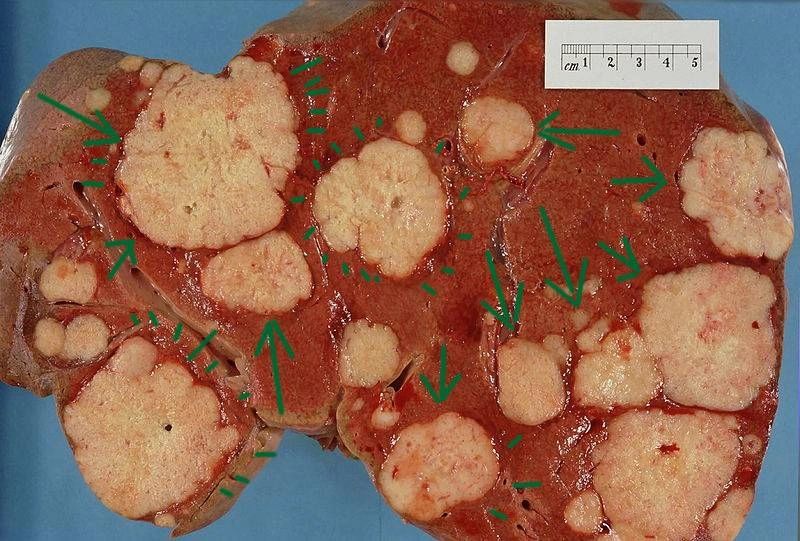
Above Left
Image: Metastatic deposits in the liver. Cross section of a human liver,
taken at autopsy examination, showing multiple large pale tumor
deposits (green arrows). The tumor is an adenocarcinoma derived from a
primary lesion in the body of the pancreas. Courtesy of Wikimedia
Commons.
Another case report involves pancreatic cancer which responded favorably to LDN.
A 46-year-old man who was diagnosed with metastatic pancreatic cancer in October 2002. He was initially treated with a standard chemotherapy regimen by the local oncologist. However, after a single chemotherapy treatment, the patient experienced severe bone marrow suppression with low platelet and WBC counts, and could not tolerate any further chemotherapy. In addition, in spite of the chemotherapy, the cancer progressed.
The patient then presented to Dr. Berkson, who promptly started treatment with intravenous Alpha Lipoic Acid (ALA), Low Dose Naltrexone (LDN), and a healthy lifestyle program. The Pancreatic cancer with metastases to the liver was followed with serial CAT and PET scans, and he has remained stable.
It is interesting to note that the cancer progressed rapidly when the ALA-LDN therapy was halted; however, the cancer stabilized quickly when treatment resumed. Serial Cat scans show no progression of hepatic metastatic lesions over three years. These can be viewed here.
A phase I clinical trial by Smith and Zagon using Opiate Growth Factor in advanced pancreatic cancer patients showed that OGF can be safely administered to patients with advanced pancreatic cancer.
Here is a videos presentation by Dr Burt Berkson, Oct 2008 at the LDN Conference at USC Medical Center: This is Part One of Three Parts.
Click here to view Parts Two and Three of Dr Berksons's LDN presentation:
Part Two
Part Three
How does LDN restrict pancreatic cancer?
Basic science research by Zagon using molecular biology tools has answered this question. Zagon found that Opioid Growth Factor and receptor inhibit growth of pancreatic cancer cells by influencing cellular replication during the G0/G1 phase of mitosis. Zagon found that this inhibition of cell replication in human pancreatic cancer involves a well known pathway in molecular biology, the p21-CKI pathway. This inhibition of cell replication can be obtained with Opioid Growth Factor itself, or with LDN.
A New Book on LDN:
 The Promise of Low Dose Naltrexone Potential Benefits in Cancer, Autoimmune, Neurological and Infectious Disorders. By Elaine Moore, co-author SammyJo Wilkinson Foreword by Dr. Yash Agrawal, MD, PhD
The Promise of Low Dose Naltrexone Potential Benefits in Cancer, Autoimmune, Neurological and Infectious Disorders. By Elaine Moore, co-author SammyJo Wilkinson Foreword by Dr. Yash Agrawal, MD, PhD Naltrexone was developed in the 1960's as part of the governments War on Drugs. Naltrexone was intended to be used as a treatment or preventive for narcotics addiction. However, it was never popular with Narcotics addicts because it causes narcotics withdrawal, a very painful and uncomfortable state. It seems to have developed a niche for treatment and prevention of alcoholism. The usual tablet dosage is 50 mg, although some practitioners use a monthly injection for alcoholics.
The book covers the use in LDN in the following disease categories:
History of LDN and use in:
Autoimmune Diseases
Multiple Sclerosis
Neurodegenerative Disorders
Cancer
Autism Spectrum Disorders
LDN in Wound Healing and Infections
The Immune System and LDN in HIV/AIDS
The LDN Experience: A Patient’s Guide to LDN
I received a courtesy copy of the new LDN book from the publisher
www.mcfarlandpub.com (call 800 253-2187 to order your copy).
This is perhaps the first, and so far only book on LDN, and as such represents a milestone in the effort to bring LDN into mainstream use. Written by Elaine Moore, a high level science writer with a portfolio of previous accomplishments, her LDN book is perhaps somewhat technical and may be difficult for the untrained non-professional to follow. It delves into the sophisticated jargon of the medical research world. For example, in Chapter 5 on LDN and Cancer, there is a discussion of Zagon's work on Cyclin dependent kinases, P53 and protein 21 and how this relates to inhibition of cancer by LDN.
However, in addition to the esoteric technical sections of the book, there are also chapters devoted to the lay reader interested in learning how LDN can help them on a practical level. A listing of dispensing practitioners was included which I found contained my own office address and phone number.
The book is highly recommended for other health care practitioners who wish to get quickly up to speed in this new area of medicine which is destined to become the medical paradigm of the 21st century, casting a giant shadow over the rest of mainstream medicine.
Buy Book on Amazon
Articles with Related Content:
Low Dose Naltrexone LDN by Jeffrey Dach MD
Jeffrey Dach MD
7450 Griffin Rd Suite 180/190
Davie, FL 33314
Phone: 954-792-4663
Blog
Links and references:
Videos of the meeting presentation by Dr Burt Berkson, a clinician using LDN, at the Oct 2008 LDN conference at USC Medical Center, in which he presents cases of successful remission from pancreatic cancer and B Cell lymphoma with LDN. He also notes most dramatic responses in cases of autoimmune diseases such as Rheumatoid Arthrisis, Systemic Lupus and Dermatipmyosists, as well as multiple sclerosis and inflammatory bowel disease.
//www.youtube.com/watch?v=WqRwXEnPYKk
October 11 2008 USC Medical Center . LDN 2008 Dr Burt Berkson Best of part 1
//www.youtube.com/watch?v=4bpRai9S03A&feature=related
LDN 08 Dr Burt Berkson Part 2
//www.youtube.com/watch?v=BLoS_U85g0Y&feature=related
LDN 2008 Dr Burt Berkson Part 3
Burt Berkson's publications of case reports of success with LDN for pancreatic cancer and B cell lymphoma:
http://www.ldn4cancer.com/files/berkson-b-cell-lymphoma-paper.pdf
Reversal of Signs and Symptoms of a B-Cell Lymphoma in a Patient Using Only Low-Dose Naltrexone. Integr Cancer Ther 2007; 6; 293
Burton M. Berkson, MD, Daniel M. Rubin, ND, FABNO, and Arthur J. Berkson, MD
PET scans before and after LDN treatment for B cell lymphonma shows dramatic resolution of enlarged cervical and inguinal lymph nodes. This case report describes the treatment of a 61-year old man with biopsy-proven Lymphoma. His initial physical examination and PET/CT scan showed multiple large, metabolically active, pathologic lymph nodes that impressively demonstrated complete resolution within 6 months of commencing therapy with nocturnally administered LDN.
http://www.ldn4cancer.com/files/Berkson_Pancreatic_paper.pdf
The Long-term Survival of a Patient With Pancreatic Cancer With Metastases to the Liver After Treatment With the Intravenous Alpha-Lipoic Acid/Low-Dose Naltrexone Protocol by Burton M. Berkson, Daniel M. Rubin, and Arthur J. Berkson
INTEGRATIVE CANCER THERAPIES 5(1); 2006 pp. 83-89
In this case report, we describe the treatment of a 46-year-old man who was diagnosed with metastatic pancreatic cancer in October 2002. He was initially surveyed and staged by a local oncology team and treated with a standard chemotherapy regimen. After a single treatment of gemcitabine and carboplatin, the patient became leukopenic and thrombocytopenic and could not tolerate any further chemotherapy. In addition, even with the standard chemotherapy protocol, his cancer progressed. J.A. then arrived at the office of one of the authors (B.M.B.) and was promptly started on a program of intravenous Alpha Lipoic Acid (ALA), Low Dose NAltrexone (LDN), and a healthy lifestyle program. During the 3 year period from October 2002 to present (December 2005), J.A.’s pancreatic cancer with metastases to the liver was followed closely by regular office visits and CT and PET scans, and he has remained mostly stable (Figure 7). It is interesting to note that J.A.’s disease progressed rapidly when he went off the ALA-LDN therapy; however, it stabilized quickly when he resumed the treatment. Serial Cat scans show no progression of hepatic metastatic lesions over three years.
Basic Science Research by Zagon using Molecular Biology Tools
http://www.molecular-cancer.com/content/7/1/5
The OGF-OGFr axis utilizes the p21 pathway to restrict progression of human pancreatic cancer. Fan Cheng1 , Patricia J McLaughlin1 , Michael F Verderame2 and Ian S Zagon Department of Neural and Behavioral Sciences and Dept of Medicine, The Pennsylvania State University College of Medicine, Hershey, PA, USA
Molecular Cancer 2008, 7:5doi:10.1186/1476-4598-7-5
Background. Pancreatic cancer is the 4th leading cause of death from cancer in the U.S. The opioid growth factor (OGF; [Met5]-enkephalin) and the OGF receptor form an inhibitory growth regulatory system involved in the pathogenesis and treatment of pancreatic cancer. The OGF-OGFr axis influences the G0/G1 phase of the cell cycle. In this investigation, we elucidate the pathway of OGF in the cell cycle.
Results. OGF treatment increased cyclin-dependent kinase inhibitor (CKI) p21 protein expression in comparison to controls, as well levels of p21 complexed with Cdk2. Naloxone abolished the increased expression of p21 protein by OGF, suggesting a receptor-mediated activity.
p21 specific siRNAs blocked OGF's repressive action on proliferation in BxPC-3, PANC-1, and Capan-2 cells; cells transfected with negative control siRNA had no alteration in p21 expression, and therefore were inhibited by OGF.
Conclusion.These data are the first to reveal that the target of cell proliferative inhibitory action of OGF in human pancreatic cancer is a p21 CKI pathway, expanding strategies for diagnosis and treatment of these neoplasias.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2613082&tool=pmcentrez
Mol Biol Cell. 2009 January 1; 20(1): 319–327.
The OGF–OGFr Axis Utilizes the p16INK4a and p21WAF1/CIP1 Pathways to Restrict Normal Cell Proliferation
Fan Cheng,* Patricia J. McLaughlin,* Michael F. Verderame,† and Ian S. Zagon* *Department of Neural and Behavioral Sciences, and †Department of Medicine, The Pennsylvania State University College of Medicine, Hershey, PA 17033 Jonathan Chernoff, Monitoring Editor
Opioid growth factor (OGF) is an endogenous opioid peptide ([Met5]enkephalin) that interacts with the OGF receptor (OGFr) and serves as a tonically active negative growth factor in cell proliferation of normal cells. To clarify the mechanism by which OGF inhibits cell replication in normal cells, we investigated the effect of the OGF–OGFr axis on cell cycle activity in human umbilical vein endothelial cells (HUVECs) and human epidermal keratinocytes (NHEKs). OGF markedly depressed cell proliferation of both cell lines by up to 40% of sterile water controls. Peptide treatment induced cyclin-dependent kinase inhibitor (CKI) p16INK4a protein expression and p21WAF1/CIP1 protein expression in HUVECs and NHEKs, but had no effect on p15, p18, p19, or p27 protein expression in either cell type. Inhibition of either p16INK4a or p21WAF1/CIP1 activation by specific siRNAs blocked OGF inhibitory action. Human dermal fibroblasts and mesenchymal stem cells also showed a similar dependence of OGF action on p16INK4a and p21WAF1/CIP1. Collectively, these results indicate that both p16INK4a and p21WAF1/CIP1 are required for the OGF–OGFr axis to inhibit cell proliferation in normal cells.
In conclusion, our results support the notion that both p16INK4a and p21WAF1/CIP1 act as suppressors to mediate the growth inhibitory function of the OGF–OGFr axis in normal cells. The clinical ramifications of our findings merit further discussion. For example, drugs used to attenuate p16INK4a or p21WAF1/CIP1 and to decrease OGF–OGFr interfacing would have the net effect of increasing cell proliferation and accelerating processes dependent on cell production (e.g., wound healing). Indeed, topical and systemic application of the opioid antagonist naltrexone stimulates wound repair of ocular surface epithelium in normal and diabetic rats (Zagon et al., 2007b ). Increasing both p16INK4a and p21WAF1/CIP1 would be predicted to have an additive effect in concert with the OGF–OGFr axis to slow down cell proliferation. This could have an impact in situations wherein retardation in the generation of cell number would be desirable. For example, in cases of hyperplasia such as endometrial hyperplasia or benign prostatic hyperplasia, cell proliferation would be decreased by increasing p16INK4a and p21WAF1/CIP1 in concert with activating the OGF–OGFr system.
http://www.ncbi.nlm.nih.gov/pubmed/18813788
Int J Oncol. 2008 Oct;33(4):751-7. Prevention and delay in progression of human squamous cell carcinoma of the head and neck in nude mice by stable overexpression of the opioid growth factor receptor.
McLaughlin PJ, Kreiner S, Morgan CR, Zagon IS. Department of Neural and Behavioral Sciences, The Pennsylvania State University College of Medicine, Hershey, PA, USA.
http://www.ncbi.nlm.nih.gov/pubmed/18636152
Int J Oncol. 2008 Aug;33(2):317-23. Prevention and delay in progression of human pancreatic cancer by stable overexpression of the opioid growth factor receptor.
Zagon IS, Kreiner S, Heslop JJ, Conway AB, Morgan CR, McLaughlin PJ.
Department of Neural and Behavioral Sciences, The Pennsylvania State University College of Medicine, Hershey, PA 17033, USA.
This study examined overexpression of the opioid growth factor receptor (OGFr) in pancreatic cancer cells and phenotypic changes in tumorigenicity. Tumors of MIA PaCa-2 cells transfected with OGFr cDNA (OGFr-1) had 3.3 times more OGFr than empty vector (EV) neoplasias, and 4.3 times more OGFr than tumors from wild-type (WT) mice. No differences in OGFr binding were detected between tumors of EV and WT animals. Tumor incidence in OGFr-1 animals was reduced by up to 50% from EV mice. Latency times for OGFr-1 tumor expression were increased 30%, tumor volume was decreased 70%, and DNA synthesis was reduced 24% relative to EV mice. Exogenous OGF reduced OGFr-1 tumor volume up to 55% compared to OGFr-1 mice given vehicle. These data support OGFr gene function as a regulator of cell proliferation that impacts on tumorigenic expression, and suggest that molecular and pharmacological manipulation of OGFr may prevent or delay human pancreatic cancer.
http://www.ncbi.nlm.nih.gov/pubmed/15014352
Anticancer Drugs. 2004 Mar;15(3):203-9. Treatment of advanced pancreatic cancer with opioid growth factor: phase I. Smith JP, Conter RL, Bingaman SI, Harvey HA, Mauger DT, Ahmad M, Demers LM, Stanley WB, McLaughlin PJ, Zagon IS.Department of Medicine, Pennsylvania State University College of Medicine, Hershey, PA 17033, USA.
Opioid growth factor (OGF) is an endogenous pentapeptide that inhibits growth of human pancreatic cancer cells in culture, as well as xenografts in nude mice. To establish the maximum tolerated dose (MTD), and determine safety and toxicity of OGF, a phase I trial was performed in patients with advanced unresectable pancreatic cancer. Patients with unresectable pancreatic adenocarcinoma were treated with escalating doses of OGF for 30 min i.v. to determine the MTD. The s.c. route of administration also was evaluated. Once the MTD was established, a group of patients was treated chronically, and monitored for safety and toxicity. Hypotension was the dose-limiting toxicity, resulting in a MTD of 250 microg/kg i.v. Due to limited solubility of OGF in small volumes, a maximum dose of 50 microg/kg twice daily was determined by the s.c. route of administration. No adverse events were reported for oxygen saturation, cardiac rhythm, laboratory values or neurological status in either the acute or chronic parts of the study with the i.v. or s.c. routes. During the chronic i.v. phase, two subjects had resolution of liver metastases and one showed regression of the pancreatic tumor. Mean survival from the time of diagnosis was 8.7 months (range 2-23 months) in the i.v. group and 9.5 months (range 1-18 months) in the s.c. group. We conclude that OGF can be safely administered to patients with advanced pancreatic cancer. Further studies are needed to determine the efficacy of OGF alone or in combination with present modes of therapy for the treatment of pancreatic cancer.
http://www.ncbi.nlm.nih.gov/pubmed/8853403
Am J Physiol. 1996 Sep;271(3 Pt 2):R780-6. Links
Opioid growth factor ([Met5]enkephalin) prevents the incidence and retards the growth of human colon cancer.Zagon IS, Hytrek SD, Lang CM, Smith JP, McGarrity TJ, Wu Y, McLaughlin PJ.Department of Neuroscience and Anatomy, Pennsylvania State University, College of Medicine, Hershey 17033, USA.
Endogenous opioid peptides serve as growth factors in normal and neoplastic cells and tissues, and both opioids and their receptors have been identified in human colon cancer. This study examined the hypothesis that opioids serve to modulate the growth of human colon cancer.
Daily administration of the native opioid growth factor (OGF), [Met5]enkephalin, at dosages of 0.5, 5, or 25 mg/kg prevented the occurrence of human colon cancer HT-29 xenografts in nude mice. More than 80% of the mice receiving OGF beginning at the time of tumor cell inoculation did not exhibit neoplasias within 3 wk, in comparison with a tumor incidence of 93% in control subject. Even 7 wk after cancer cell inoculation, 57% of the mice given OGF did not display a tumor. OGF delayed tumor appearance and growth in animals developing colon cancer with respect to the control group. The suppressive effects of OGF on oncogenicity were opioid receptor mediated. OGF and its receptor, zeta (zeta), were detected in transplanted human HT-29 colon tumors. Surgical specimens of human colon cancers also contained OGF. These results show that a naturally occurring opioid peptide acts as a potent negative regulator of human gastrointestinal cancer and may suggest pathways for tumor etiology, progression, treatment, and prophylaxis.
4th Annual LDn Conference 2008 You Tube Videos of Presentations
//www.youtube.com/watch?v=ftykRVq76BA
&feature=player_embedded
//www.youtube.com/watch?v=UNX3eeg4c_I&feature=player_embedded
4th Annual LDn COnference. LDN 2008 Sunny Sedlock O’Malley Interview
corrdinator of 2008 meeting discusses how her fathers Multiple Myeloma went into remission with LDN
//www.youtube.com/watch?v=DAZ1fQKdOC8&feature=player_embedded
3rd LDN Conference Doctor Interviews 2007. Dr David Gluck gives overview of conference. 2007 3rd.
ldn for UC Ulcerative Colitis
http://www.drhoffman.com/page.cfm/795
One patient's UC success story. Nick's success story
I was diagnosed with Ulcerative Colitis just a few days after my 16th birthday. Today, at age 18, thanks to Dr. Hoffman, and the rest of the Staff at the Hoffman Center, I have become 100% symptom free through diet, supplementation and Low Dose Naltrexone. My colitis was severe. I was anemic, I had lost almost 30 pounds, I could not participate in high school sports, my attendance at school was very sporadic and I was on the verge of surgery to have my colon removed.
As a last hope, my parents took me to the Hoffman Center as they were familiar with Dr. Hoffman's "intelligent medicine" from his radio show. My parents believed there had to be an answer other than the Remicade and steroids my second Gastroenterologist had prescribed for me. Both my first GI doctor, who had me on 19 Asacol pills per day, and my second GI doctor, who had me on the very dangerous immune suppressing drug Remicade, maintained diet had nothing to do with my colitis.
Dr. Hoffman, though, immediately put me on the Specific Carbohydrate Diet (SCD). With guidance from the Hoffman Center Nutritionist, Leyla Muedin, I have been able to stick to the diet despite my initial concerns about always being hungry, or not getting enough calories to gain weight or have energy.
The second thing Dr. Hoffman did was to set up a plan of supplements for me to take without overwhelming me with a 100 pills a day. It's simple and easy to maintain, and my parents are able to get all the supplements from the Hoffman Center through the mail.
To help boost my nutritional intake, I was also getting 2 or 3 "IV" treatments per month at the Hoffman Center. I am now down to 1 "IV" treatment per month just for maintenance.
The third part of the successful treatment plan Dr. Hoffman established for me was a prescription for the medication known as Low Dose Naltrexone, or LDN for short. Dr. Hoffman wrote the prescription for LDN in late May of 2007 based upon his research of a study performed by Dr. Jill Smith at Hershey Medical Center. Doctor Hoffman learned of the study when the results were published in a national GI medical journal. Unlike Remicade, which has the possible side affect of lymphoma because it limits the immune system, LDN improves the immune system.
In September of 2007, my parents took me to see Dr. Smith who recommended I continue the protocol Dr. Hoffman has prescribed. For the last 5 months, I have not had a single symptom of my colitis, and I am no longer anemic. In the 7 months of following Dr. Hoffman's plan, my health, strength and energy are as strong as they ever have been. I have gained nearly 35 pounds, my attendance at school is 100%, and for the first time since freshman year, I have been able to run track and cross country without missing an event.
Before seeing Dr. Hoffman, I was confined to the house except for when I tried to go to school or had a doctor's appointment. I couldn't work or do anything social with friends. Most days I couldn't make it up the stairs to my bedroom. I was in the hospital emergency room 2 times just in the first few months of 2007 because of pain and dehydration.
While it was nearly impossible for my parents to get my second GI doctor to return a phone call, Dr. Hoffman or his staff would not let the evening pass before they returned a call and were speaking to my mom or dad.
When I saw my GI doctor in July of 2007, he refused to acknowledge the benefits of the SCD diet, supplementation or LDN. He dismissed the study Dr. Smith wrote about and Dr. Hoffman believed in.
Instead, since it had not helped at all, he wanted to increase the dosage and frequency of infusion of the Remicade. My parents and I walked out, and we haven't spoken to him since. From my first visit to the Hoffman Center, the change in my life - which really is like having life back, has been amazing. I owe it to Dr. Hoffman and the Staff at his Center.
Nick P. Doylestown, PA January 2008
http://www.ldners.org/index.htm
News about LDN
http://www.webspawner.com/users/ldnforcancer/index.html
Treating Cancer With Low Dose Naltrexone (LDN)
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Florida 33314
954-792-4663
http://www.drdach.com/
http://www.naturalmedicine101.com/
http://www.truemedmd.com/
http://www.bioidenticalhormones101.com/
Disclaimer click here: http://www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with
his/her personal physicians and to only act upon the advice of his/her
personal physician. Also note that concerning an answer which appears as
an electronically posted question, I am NOT creating a physician —
patient relationship.
Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2014 Jeffrey Dach MD All Rights Reserved
This article may be reproduced on the internet without permission,
provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation